ASTar instrument
The fully automated ASTar instrument provides robust and consistent inoculum preparation for AST, with high-speed time-lapse microscopy imaging of bacteria in broth for MIC determination. ASTar can be combined with any of the rapid ID technologies, which augments current laboratory capabilities and meets the clinical need for more rapid results.

Add positive blood culture and load disposables
The process begins with the user transferring an aliquot of the patient sample to the sample preparation cartridge.
The user then choses a suitable AST panel disc that is loaded in the instrument, the barcode on the disk is scanned automatically during loading.
The cartridge barcodes, for the patient sample and cartridge respectively, are scanned by a reader after which the cartridge is loaded in the instrument.
After this all required steps are carried out automatically by ASTar.

Automated sample preparation
The instrument automatically isolates intact and viable bacteria from the sample and the isolated bacteria are placed in a suitable growth medium for subsequent AST.
The system then measures the concentration of the isolated bacteria. Based on this measurement, bacteria are diluted in a suitable growth medium to produce an appropriate inoculum for AST. A controlled inoculum is an important prerequisite for obtaining stable data, the risk otherwise is that the MIC value could be affected by the bacteria concentration, producing incorrect results.
A subset of the sample is also diluted in a separately enriched growth medium to facilitate AST of organisms that require special growth conditions (fastidious organisms). The capacity to simultaneously handle both types of organisms means that the analysis can begin without knowing bacterial ID, which saves time. The two different inoccula are loaded into culturing chambers prefilled with antimicrobials, through centralized filling ports in the disc by the instrument’s pipetting robot.

AST analysis
The AST disc is incubated in a temperature-controlled part of the instrument and the culturing chambers are read at even intervals by a high-speed, high-resolution optical detection system.
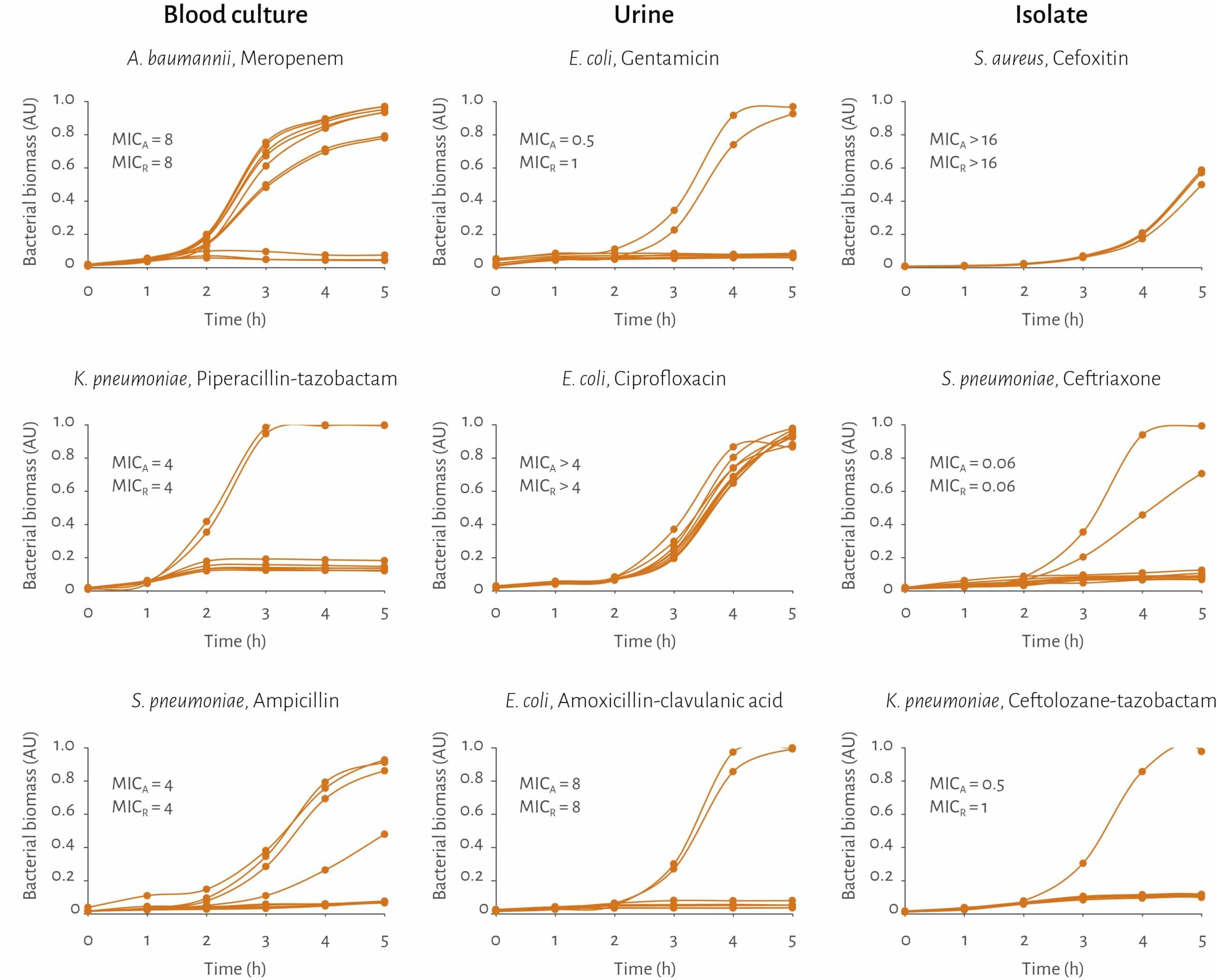
An image analysis algorithm continuously evaluates the collected images to quantify the accumulated bacterial biomass in the culturing chambers. When the incubation is completed, time-dependent bacterial biomass curves are compiled for each antimicrobial type and concentration.
The analysis can continue without knowing the bacterial ID up until this point. When the ID is available, MIC is calculated for each antimicrobial by a separate algorithm that also weighs in information about the bacterial ID. Using this value as the basis, bacteria are classified as susceptible (S), intermediate (I) or resistant (R) in terms of the current antimicrobials.
ASTar facilitates a wide concentration range for each antimicrobials, which ensures sufficient coverage around the breakpoints, even if they are updated.
Ready to explore the AST solution for all needs?
Connect with us!
The AST technology has been extensively tested on clinically relevant pathogens in correct sample matrix
✓ Over 380 different strains including several challenge strains
– 25 different species
– Gram positive and Gram negative bacteria
– Fastidious pathogens
✓ 30 different antimicrobials
✓ In total over 5900 bug-drug combinations to date – steadily increasing

Designed to simplify


